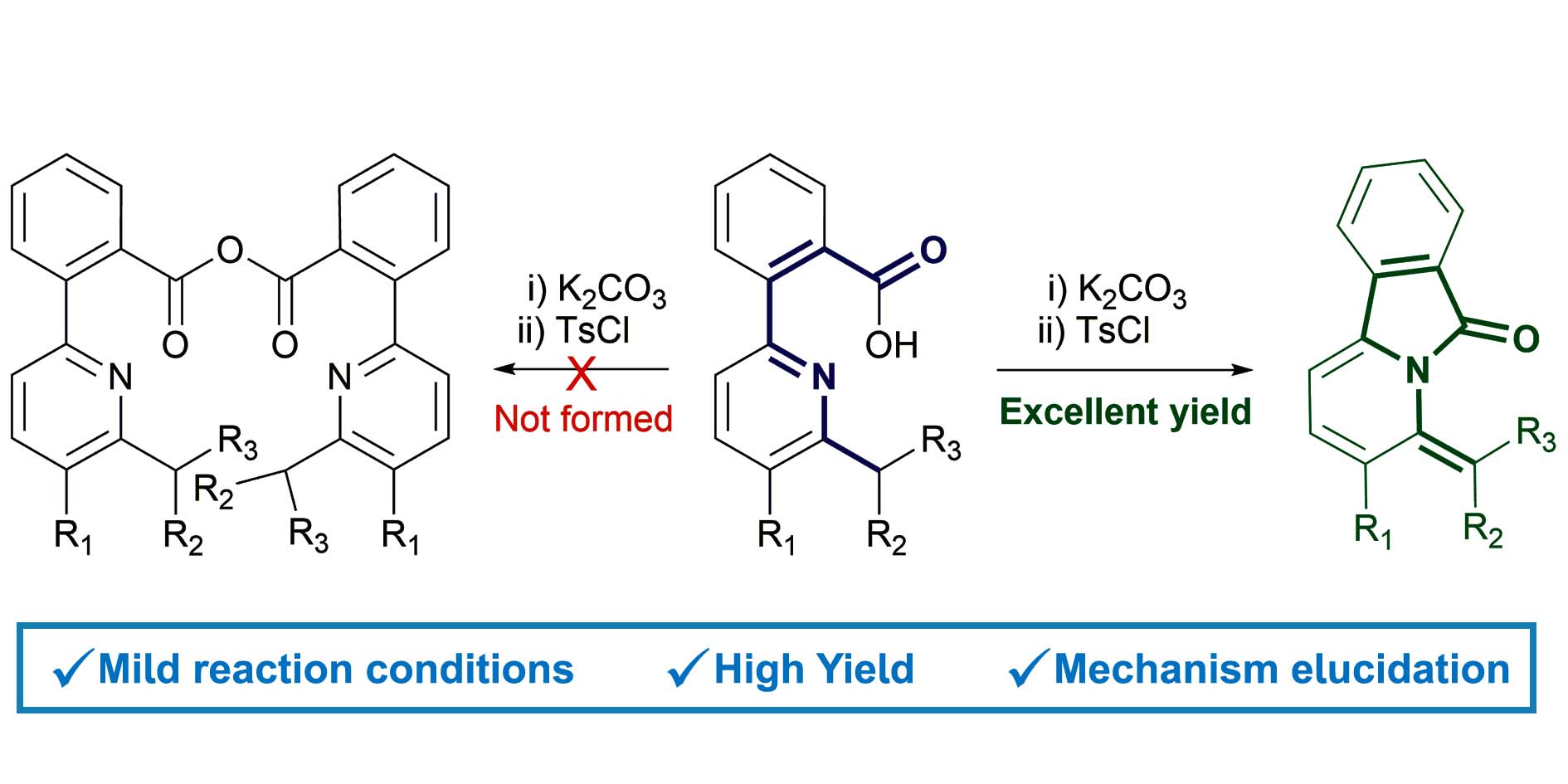
Efficient synthesis of isoindolones by intramolecular cyclisation of pyridinylbenzoic acids
A straightforward one-pot method for the synthesis of unreported pyrido-[2,1-a]isoindolones in excellent yield is described. Two novel isoindolones were synthesized and fully characterized. The alkyl substituents on the pyridine play an important role in the outcome of the reaction. The mechanism, investigated through DFT calculations, features an unprecendented intramolecular cyclization reaction involving a carboxylic acid activated by tosyl chloride and an electron-poor pyridinic nitrogen. This protocol completes the known strategies to obtain functionalized isoindolones.
This paper was published in collaboration with Atena B. Solea, Sining Wang, Xiao-Song Xue, Aurelien Crochet, Katharina M. Fromm, Kendall N. Houk and Olimpia Mamula.
To read the entire paper, clic here.